Immunotoxicology Laboratory - Dr. Jared Brown
Research in the Brown lab is focused on immune responses to particulates including nanoparticles, air pollution, silica and coal dust. In particular, our research group investigates the role of innate immune cells, including mast cells and macrophages, in response to particulates and their role in disease. In addition, we are interested in understanding the toxicity of engineered nanomaterials as well as their use in drug delivery and treatment of cancer. Lastly, we are interested in understanding the contribution of environmental contaminants in the development of chronic kidney disease of unknown origin (CKDu).
Jared Brown PhD
Professor
T32 Training Program in Molecular and Systems Toxicology Director, Colorado Center for Nanomedicine and Nanosafety Co-Director
Email:[email protected]
Phone:303-724-8213
Research in the Brown lab broadly focuses on immune responses to environmental and occupational exposures. Our research ranges from understanding immune responses to nanoparticles and air pollution to chemical warfare agents and occupational exposures. In addition, we are interested in developing novel nanotherapeutics for treatment of cancer and allergic disease. Examples of current projects in the Brown lab are described below:
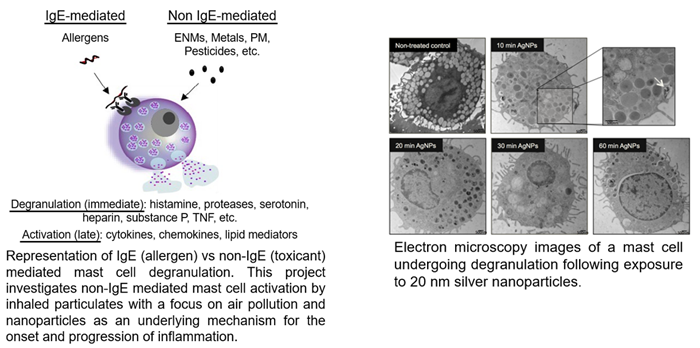
1. Understanding mechanisms of non-IgE mast cell activation by environmental particulates.
This work, funded by NIEHS R01 ES019311, is examining novel mechanisms by which nanoparticles and airborne particulate matter trigger non-IgE mast cell activation contributing to adverse pulmonary and cardiovascular outcomes. Specifically, we are investigating redox and non-redox mechanisms linked with thioredoxin interacting protein and its potential regulatory role in mast cell degranulation. In addition, we are interested in cellular metabolism changes which occur between IgE and non-IgE mast cell degranulation that may provide insight into disease mechanisms in mast cell activation disorders. Lastly, we are working with Drs. Stephen Dreskin and Jenny Stitt from the Anschutz School of Medicine to examine these mechanisms of non-IgE mast cell degranulation in patients with chronic idiopathic urticaria.
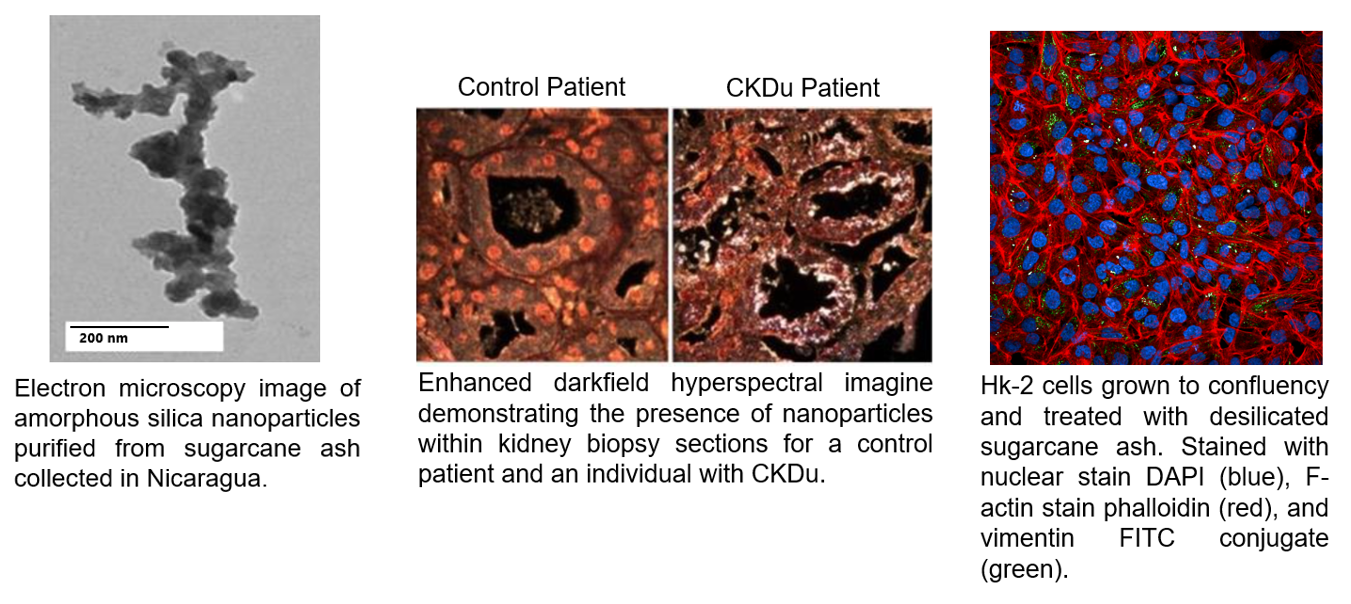
2. Silica Nephropathy and Chronic Kidney Disease of Unknown Etiology.
This work, funded by NIDDK R01 DK12351 and in collaboration with Drs. Richard Johnson and Carlos Roncal within the School of Medicine, is examining the contribution of inhaled silica nanoparticles produced during burning of sugarcane fields in the development of chronic kidney disease of unknown etiology (CKDu). CKDu is worldwide epidemic largely associated with coastal agricultural workers leading to chronic kidney disease in young individuals and often resulting in kidney transplant or death. There are many potential contributing factors including heat stress, dehydration and environmental exposures. We are currently examining kidney biopsy sections from CKDu patients for the presence of silica nanoparticles that are found in high abundance in the ash from sugarcane burning. We are using two novel techniques to identify silica nanoparticles in biopsy sections: 1) single particle inductively coupled plasma mass spectrometry to provide size and chemical information on the silica nanoparticles and 2) enhanced darkfield hyperspectral imaging to image nanoparticles in tissue. In addition, we are using a human proximal convoluted tubule cells and animal models to investigate potential mechanisms of toxicity by sugarcane ash and purified amorphous silica nanoparticles.
3. Contribution of mast cells to nitrogen mustard pulmonary and central nervous system toxicity.
This work, funded by the NIEHS and the Department of Defense and working with Dr. Neera Tewari-Singh (Michigan State University), is investigating the role of mast cells in mediating inflammation in response to exposure to sulphur mustard and phosgene oxime, both of which are chemical warfare agents. We have utilized a mouse model of mast cell deficiency to demonstrate that in the absence of mast cells that inflammation resulting from sulphur mustard exposure is largely diminished. In addition, we are examining the effects of mast cell activation in the brain following sulphur mustard exposure. Lastly, we developing a nanoparticle based therapeutic to prevent mast cell activation as a prophylactic treatment for military personnel and civilians to prevent the effects of chemical warfare agents.
.tmb-image350.png?Culture=en&sfvrsn=55c507bb_1)
4. Inductively coupled plasma mass spectrometry studies:
Our lab employs ICP-MS to investigate environmental metal exposures across human, animal, and cellular samples. Through several funded collaborations with multiple investigators, we analyze trace metal concentrations to assess exposure risks and biological impacts. Utilizing our NexION 2000 ICP Mass Spectrometer, we have developed a distinct single particle method to both measure nanoparticles and identify individual metals.
Angela Reinert, MS
Graduate Student
[email protected]
Angela is studying in the Toxicology PhD program. She is investigating IgE-independent mast cell activation pathways activated by environmental toxicant exposure with a specific interest in the Mas-Related G Protein-Coupled Receptor X2 (MRGPRX2).

Robert Canfield
Graduate Student
[email protected]
Bob is studying in the Pharmaceutical Sciences PhD program. He is helping develop a phage-like nanoparticle for the treatment of bladder cancer. Specifically, he is investigating activation of the STING pathway to induce an anti-tumor immune response by the phage like nanoparticles.

Carly Chesterman, MS
Graduate Student
C[email protected]
Carly is studying in the Toxicology PhD program. She is researching how environmental toxicant exposure may contribute to the development of chronic kidney disease of unknown etiology. Her current work relates to the potential synergistic toxicity of common pesticides and sugarcane ash in the context of renal disease.

Sebastián Santos, BSc
Graduate Student
[email protected]
Sebastián is studying in the Pharmaceutical Sciences Master’s program following the Toxicology tract. He helps with method development and routine analysis of elemental and nanoparticle identification in biological and environmental samples using ICP-MS. ">

Matthew Gibb, MS, PhD
Post-Doctoral Fellow
[email protected]
Matthew has previously worked in pulmonary immune responses to infectious diseases, and recently finished his PhD in pulmonary immunotoxicology where he worked on using in vitro models to identify and differentiate respirable sensitizers from non-sensitizers. Currently, he is investigating pathways and mechanisms involved in CKDu from environmental exposures as well as looking into the role mast cells play in response to inhaled chemical warfare agents.

Stephen Brindley, MS
Professional Research Assistant III
[email protected]
Stephen runs the ICP-MS instrument where he quantifies metals in prenatal vitamins and analyzes levels of various nanoparticles in different biological matrices. Stephen also works on a coal related project evaluating if particle size plays a role in coal toxicity.

| Lab Alumni | Current Position |
| Arthur Stem, PhD | Post-Doctoral Fellow, Yale University |
| Diksha Diundi, MS | Regulatory Fellow at Synterex |
| Yanhao Jiang, MS | Graduate Student in Pharmaceutical Sciences at University of Arizona |
| Keegan Rogers, PhD | Senior Environmental Scientist, Ahtna Solutions LLC |
| Angela A. Cruz, PhD | Senior Scientist – Clinical Innovation (Advanced Research), L’Oréal |
| Ryan Mendoza, PhD | Research Scientist, Bio-Rad Laboratories |
| Dylan Fudge, PhD | Molecular Toxicologist, U.S. Army DEVCOM Chemical Biological Center |
| Nasser Alsaleh, PhD | Associate Professor, King Saud University |
| Indushekhar Persaud, PhD | Principal Investigator/Associate Director, Incyte Pharmaceuticals |
| Valerie Minarchick, PhD | Research Associate, University of Colorado Anschutz Medical Campus |
| Jonathan Shannahan, PhD | Associate Professor, Purdue University |
| Monica Johnson, PhD | Co-Founder, STEM Boomerang, New Mexico |
| Wei Bai, PhD | Owner, SilkRdConnect, Scientific Communications |
| Ramakrishna Podila, PhD | Associate Professor, Clemson University |
| Sky (Xiaojia) Wang, PhD | Professor, Beaufort Community College |
| Abdullah Aldossari, PhD | Assistant Professor, King Saud University |
| Pranita Kabadi, PhD | Associate Director, Patient Safety, AstraZeneca |
