The Patel Lab
Laboratory training environment: The Patel laboratory has received 25 years of continuous NIH funding as well as funding from research organizations. Dr. Patel is a member of the MSTP, Toxicology and Neuroscience Graduate Training Programs. She served as Chair of the Graduate Training Committee for the UCD Neuroscience Training Program. She has ~20 years of experience in training postdoctoral fellows and graduate students including several underrepresented minorities (URMs). Trainees in the laboratory receive rigorous and comprehensive training in neurochemistry, metabolism, mitochondrial biology, neuroprotection, whole animal and cell culture studies, analytical methods including HPLC-UV/EC, enzymology, free radical methods, drug discovery, statistical analysis and manuscript/grant-writing/presentations. Dr. Patel is committed to training graduate students and postdoctoral researchers in basic and translational neuroscience.
Manisha Patel PhD
Professor
Associate Dean for Research and Graduate Studies
Email:[email protected]
Phone:303-724-3604
Journal of Neuroscience
Vol. 32, Issue 33
15 Aug 2012
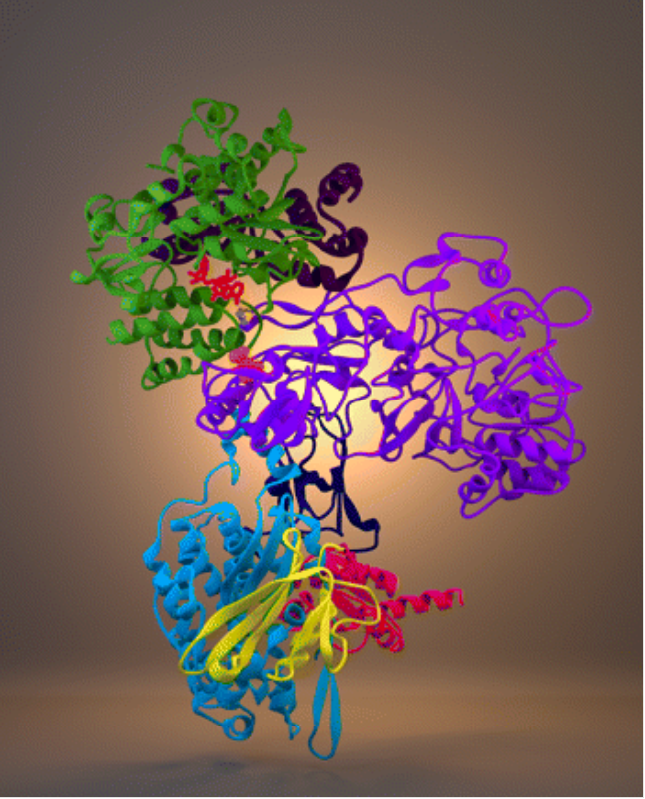
Cover legend: The hydrophilic domain of rat mitochondrial electron transport chain complex I is involved in the production of cellular ATP. During epileptogenesis, arginine 76 (orange spheres) of the 75 kDa subunit (purple ribbon) is irreversibly modified by carbonylation, leading to decreased complex I activity. This residue is located at the interaction interface between the 75 kDa subunit and the 51 kDa subunit (green ribbons), proximal to the NADH binding site (red sticks) and the sulfur-iron center involved in the initial transfer of electrons into the chain (yellow and purple sticks). For more information, see the article by Ryan et al. (pages 11250–11258).
Summary: Dr. Patel’s early work addressed a fundamental question in the field of neuroscience; namely, how does excessive glutamate neurotransmission induce excitotoxic cell death? She demonstrated a role for intracellular superoxide radicals in excitotoxic cell death emphasizing the overlapping roles of oxidative stress and excitotoxic injury, two independent pathological mechanisms common to diverse neurological disorders (Patel et al., Neuron 16:345-355, 1996). This discovery led to the overarching theme of her laboratory, which is to understand the role of reactive species and metabolic mechanisms in neuronal disorders such as epilepsy.
Research Areas. The overarching theme of the Patel laboratory is to understand the role of redox processes and metabolic mechanisms in epilepsy, aging and toxicant-induced brain injury. Using biochemical, metabolic, transgenic and translational approaches, research in the laboratory is focused on three major areas: a) understanding the mechanisms of redox and metabolic dysfunction in response to epileptogenic insults, b) developing neuroprotective drugs and therapies and c) identifying metabolic targets of ketogenic diets. The following sections describe the major research areas in the Patel lab.
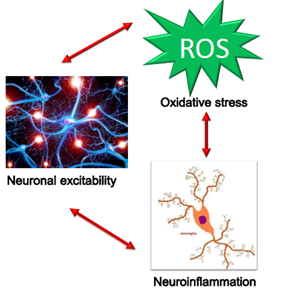
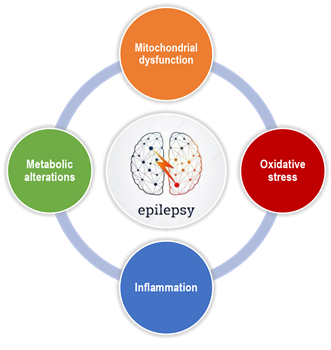
Defining the role of oxidative stress and metabolic dysfunction in epilepsy and its comorbidities. Epilepsy is a recent addition to the diverse array of acute and chronic neurological disorders in which the role of oxidative stress and mitochondrial dysfunction is rapidly emerging. Our research has been pioneering and instrumental in defining the role of redox and metabolic mechanisms in the pathogenesis of epilepsy and its comorbidities. The laboratory identified subcellular sources of reactive species (RS), their temporal occurrence and functional impact in animal models of temporal lobe epilepsy (TLE). Our focus on mitochondria arose from investigation of subcellular sources of seizure-induced RS, which indicated a disproportionate contribution of mitochondria. Work from our laboratory has demonstrated increased production of mitochondrial reactive oxygen species (mtROS) and persistent depletion of glutathione during epileptogenesis in two animal models. We demonstrated for the first time an oxidative post-translational modification via carbonylation of the 75kDa subunit of CI in experimental TLE. A functional consequence of oxidative damage to mitochondria in epilepsy is a bioenergetics decline, which can increase neuronal excitability. We recently demonstrated that increased ROS indeed result in deficits of mitochondrial respiration in experimental TLE. Finally, we showed that mitochondrial oxidative stress results in mitochondrial dysfunction and neuronal death which contributes to cognitive deficits associated with chronic epilepsy. Our studies suggest redox and mitochondrial pathways as novel therapeutic approaches for modifying acquired epilepsy and associated cognitive deficits. A recent collaboration with Dr. Scott Baraban’s laboratory identified metabolic deficits in genetic models of epilepsy, notably Dravet’s syndrome.
Delineating mitochondrial mechanisms of oxidative brain injury. Mitochondrial oxidative stress is well known to play a pathogenic role in neuronal disorders. However, the mechanisms by which ROS produce damage are incompletely understood. Our research has demonstrated that that superoxide toxicity via oxidative inactivation of mitochondrial aconitase contributes to neuronal death in animal models of neurodegeneration. Studies in the laboratory have identified a novel pool of mitochondrial iron that correlates with mitochondrial aconitase inactivation in brain injury models. We identified mitochondrial complex III as a major source of ROS via redox cycling agents. These studies led to the novel discovery that brain mitochondria consume hydrogen peroxide (H2O2) in a substrate-dependent manner via the thioredoxin/peroxiredoxin pathway linked by mitochondrial nicotinamide transhydrogenase activity.
Currently, my research involves discovering and characterizing novel phenotypes associated with mice that have neuronal specific Scn1a and SOD2 gene deletions, especially those phenotypes associated with neuronal hyper-excitability and excitotoxic cell death and changes in metabolic function. Additionally, I am involved in a clinical project utilizing lymphoblast cell lines derived from Dravet syndrome patients to examine transcriptome-wide changes in gene expression via RNA-seq analysis. In my free time, I enjoy reminding my coworkers to complete their health and safety training, taking long walks on the beach, and wondering why my data looks like that.
|
Ruth Fulton |
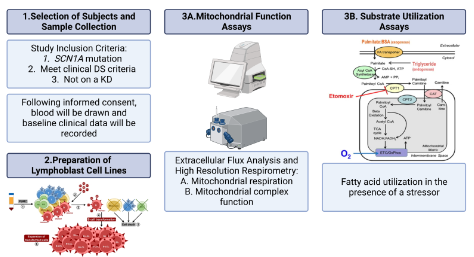
I earned my bachelor’s degree in Neuroscience and Cognitive Science from the University of Arizona, where I studied the role of extracellular vesicle mediated signaling in age-related macular degeneration (AMD) angiogenesis. I then continued to conduct research and led a pilot study to evaluate whether we can stop visual decline in patients with nAMD. The desire to learn more about translational research as it applies to drug discovery and delivery brought me to the University of Colorado where I am working on completing a PharmD/PhD under the mentorship of Dr. Patel. My research goal is to better understand seizure susceptibility by exploring the relationship between neuronal hyperexcitability, oxidative stress, and metabolic alterations. More specifically, I am currently working on a project that investigates the mechanism by which neuronal hyperexcitability is induced and exacerbated in vitro. In addition to a translational project that explores the bioenergetic profiles of cells from the systemic circulation of children with Dravet syndrome and healthy volunteers, with the goal of identifying key metabolic targets to assess patient treatment response and/or explore novel therapeutics. In my free time, I like to go to concerts and spend time with family and friends. |
Anna Figueroa |
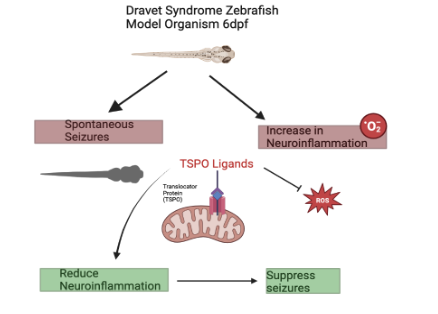
I earned my bachelor’s degree in Biomedical sciences with minors in Chemistry and Political Science at University of Northern Colorado. While there I worked on a variety of research projects, with my latest being examining snake venom metalloproteinases (SVMPs) of Crotalus oreganus lutosus. My undergraduate experience highlighted my passion for translational research, which led me to Dr. Patel’s lab. My current research is focused on neuroinflammation within Dravet Syndrome. More specifically I examine TSPO which is a biomarker for neuroinflammation. I am interested in TSPO’s mechanism of action as well as drug therapeutics that are ligands for TSPO to potentially attenuate neuronal hyperexcitability. My model organism is zebrafish larvae that are genetically modified to have a mutation in the scn1a gene, modeling Dravet syndrome.
In my free time you can find me on a river fly-fishing, trying to cook a new meal from scratch, or playing with snakes. Meet Pesto, the sweet boa constrictor who likes to roam around my hats while I type up my lab reports! |
Lauryn Adair |
| I earned my bachelor’s degree in forensic science with minors in biochemistry and biology from the Interamerican University of Puerto Rico. During my time there I worked on various research projects, all of which had a focus on toxicology and drug delivery systems. My undergraduate research experience inspired a passion for research, which led me to pursue a PhD in Toxicology. In June 2023, I officially became a part of Dr. Patel's team, where I focus on investigating the role of reactive oxygen species on initiating astrogliosis and the role of post-translational modifications of glial fibrillary acidic protein (GFAP) in influencing the severity of astrogliosis and its impact on disease. In my free time I enjoy catching up on sleep, watching movies and cooking. |
Paola Garcia Gonzalez |
| I earned my B.S. in Biochemistry and Molecular Biology with a minor in Environmental Toxicology from the University of California, Davis in 2021. My interest in multigenerational health implications of chemical exposure led me to join Dr. Michele La Merrill’s lab where I collaborated on several projects investigating the effects of exposure to toxicants and the molecular mechanisms through which they contribute to metabolic diseases. My current interest in neurodevelopmental toxicity led me to pursue a PhD in Toxicology in Dr. Manisha Patel’s lab where I now study the role of principal neurons in Dravet Syndrome using a novel neuron-specific Scn1a knockout mouse model. Outside of the lab I enjoy spending time with friends, playing tennis, oil painting, and thwarting the evil plans of my two diabolical cats, Checkers and Toast. |
Chanapa Mann |
| I graduated with my bachelor’s degree in Cognitive and Behavioral Neuroscience from Colorado State University where I studied psychomotor and balance deficits in type I diabetes. I am now pursuing my PhD in Neuroscience in Dr. Manisha Patel’s lab where I study the role of reactive oxygen species on astrogliosis and how it can contribute to hyperexcitability using in-vivo calcium imaging and a variety of in vitro techniques. In my free time I love to ski, listen to new music, and find the best happy hour in town. |
Ariana Crary |
| I received my B.S. in Biomedical Science from Colorado State University, with concentrations in Environmental Public Health and Microbiology and Infectious Disease. While there, I worked in the Moreno Lab investigating how infections may exacerbate neurodegeneration using a guinea pig model. This experience sparked my interest in neurotoxicology, which led me to pursue a PhD in Toxicology. Currently, in Dr. Patel's lab, I study TSPO, a marker of neuroinflammation, and utilize a zebrafish model to examine TSPO ligands as potential therapeutics for Dravet Syndrome. | .jpg?sfvrsn=23b10b4_1) Kristin Weninger |
| Name | Graduate Program | Current Position | Industry/ Government/ Academia | Contact |
| Dr. Derek Drechsel | Toxicology | Project Toxicologist | CTEH (Crisis Tested. Expert Solutions) | LinkedIn Profile |
| Dr. Julie Milder | Neuroscience | Senior Medical Science Liaison (MSL) | Greenwich Biosciences | LinkedIn Profile |
| Dr. Shane Rowley | Neuroscience | Lead Scientist, In vivo validation | Recursion Pharmaceuticals | LinkedIn Profile |
| Dr. David Cantu | Neuroscience | Associate Director, Medical Affairs | Sunovion Pharmaceuticals | LinkedIn Profile |
| Dr. Pamela Lopert | Neuroscience | Scientist, Clinical Endpoints | miRagen Therapeutics, Inc | LinkedIn Profile |
| Dr. Kristen Ryan | Toxicology | Toxicologist | National Toxicology Program/NIEHS | LinkedIn Profile |
| Dr. Jennifer Pearson-Smith | Neuroscience | Scientific Program Manager | Taconic Biosciences | LinkedIn Profile |
| Dr. Pallavi Bhuyan McElroy | Toxicology | Senior Scientist, Non-clinical safety | The Janssen Pharmaceutical Company | |
| Derek Johnson. MS | Neuroscience | Lab Manager | NIH NIDCD | LinkedIn Profile |
| Tim Fabisiak, MS | Pharmaceutical Sciences | Associate Program Manager | Becton Dickinson | LinkedIn Profile |
| Dr. Ashwini Sri Hari | Toxicology | Postdoctoral Scientist, Project Manager | University of Utah | LinkedIn Profile |
First of its Kind Study Identifies Metabolic Defects in Dravet Syndrome
New research from the University of Colorado Anschutz Medical Campus is the first to identify defects related to metabolism (how the body processes and uses energy) in lymphoblast cell lines (LCLs) derived from blood samples of children with Dravet syndrome.
Patel Receives Javits Award for her Body of Research on Neuroscience
When Manisha Patel, PhD, was notified she had been awarded the Javits Award in Neuroscience, she was honored. The Javits Award is a conditional, seven-year research grant given to scientists for their superior competence and outstanding productivity. Dr. Patel received her award for her ongoing work in neuroscience, researching the role of energy and oxidative stress in epilepsy. Her current work builds upon over twenty years of research in her own lab to identify the oxidation-reduction and metabolic mechanisms of seizure-induced brain injury. The project aims to test a new hypothesis and could help to develop novel metabolism-based therapies for epilepsy.
Patel Receives Javits Award for her Body of Research on Neuroscience
When Manisha Patel, PhD, was notified she had been awarded the Javits Award in Neuroscience, she was honored. The Javits Award is a conditional, seven-year research grant given to scientists for their superior competence and outstanding productivity. Dr. Patel received her award for her ongoing work in neuroscience, researching the role of energy and oxidative stress in epilepsy. Her current work builds upon over twenty years of research in her own lab to identify the oxidation-reduction and metabolic mechanisms of seizure-induced brain injury. The project aims to test a new hypothesis and could help to develop novel metabolism-based therapies for epilepsy.
Manisha Patel Installed as President of the American Epilepsy Society
Pharmaceutical Sciences Professor Manisha Patel, PhD, begins her term as president of the American Epilepsy Society (AES) at the conclusion of the Society’s annual meeting in Nashville, Dec. 2- 6. A medical and scientific society of 4,700 members, the AES is dedicated to advancing research and education for preventing, treating and curing epilepsy. Founded in 1946, AES is an inclusive global forum where professionals from academia, private practice, not-for-profit, government and industry can learn, share and grow to eradicate epilepsy and its consequences.
(1/9)
(2/9)
(3/9)
(4/9)
(5/9)
(6/9)
(7/9)
(8/9)
(9/9)




-(1).jpg?sfvrsn=2e50fdbb_1)