Anchordoquy Lab
I joined the faculty at the CU School of Pharmacy in 1998, and my early work focused on the stability of lipid-DNA complexes during freezing, drying, and storage. In attempting to assess “recovery” of these new pharmaceutical entities, I became interested in the mechanism by which they facilitated delivery to target cells after systemic delivery. We soon recognized that the exposure of particles to serum proteins caused massive aggregation that resulted in accumulation in the lung. In an attempt to avoid the use of PEGylated components, we used high cholesterol levels to impart resistance to protein-induced perturbations. In addition to imparting stability, we documented that cholesterol forms phase-separated lipid domains within our particles that offer an optimal location for targeting ligands. Our more recent work demonstrated that these particles were being avidly taken up by circulating immune cells that elicited a potent cytokine response upon intravenous injection. After expending considerable effort attempting to evade the immune response (with little success), we have shifted our focus toward exploiting the immunogenicity of particles to limit off-target accumulation of nanomedicines and promote tumor regression. In addition to these projects, my lab is constantly involved in multiple formulation studies (anticancer cream, eye drops, parenterals, ointments, injectable sustained release systems) that utilize small molecules to treat a variety of conditions.
Tom Anchordoquy MA, PhD
Professor
Department of Pharmaceutical Sciences
Email:[email protected]
Phone:303-724-6113
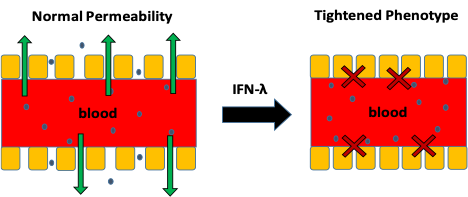
Epithelial Tightening. This project exploits the fact that gene delivery vehicles elicit an antiviral response when administered systemically, i.e., the body reacts as if it is being infected. As a mechanism of limiting the spread of infection, the body signals the vasculature to limit particle uptake. However, we have observed that the immunosuppressed state of the tumor prevents this effect, thereby allowing particles to preferentially access the tumor while reducing accumulation in non-target tissues. We have recently verified that this response only are currently characterizing this phenomenon and will utilize this to improve nanoparticle-mediated delivery to tumors.
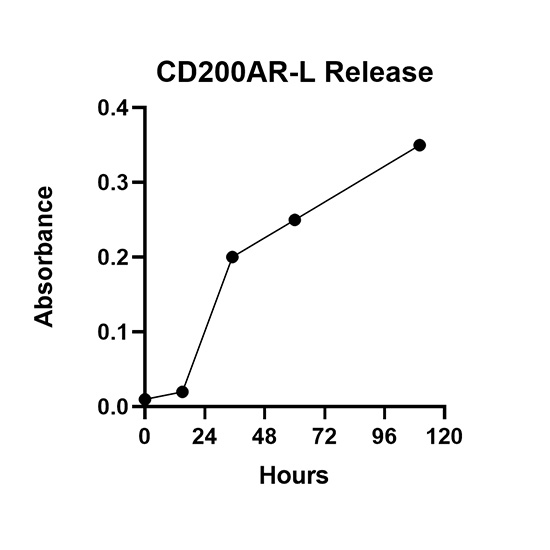
Injectable Sustained Release. This project is focused on developing a polymer-based formulation that is injected as a liquid but forms a gel under physiological conditions. The gel is composed of pharmaceutically-compatible materials that dissolve over time and are cleared from the body. By adjusting the components of the formulation we can alter the time needed for the gel to dissolve, which ultimately determines the duration of drug release. Our current project is focused on utilizing peptides for immunotherapy to treat brain tumors, but this delivery system could potentially be optimized for other drugs.
| Alumnus | Current Organization |
| Dean Allison | deceased |
| Matt Fete | Chicago State University |
| Max Kullberg | University of Alaska |
| Yvonne Lentz | Fate Therapeutics |
| Marion Molina | ILiAD Biotechnologies |
| Mayank Patel | AstraZeneca |
| Nicole Payton | Moderna Therapeutics |
| Jasmina Redzic | University of Colorado |
| Madison Ricco | University of Colorado |
| Tyson Smyth | Nektar Therapeutics |
| Scott Tilden | University of Colorado |
| Long Xu | Johnson & Johnson |
| Ye Zhang | Arcturus Therapeutics |
