Master of Science in Pharmaceutical Sciences
Through our Master of Science (MS) in Pharmaceutical Sciences program, you will be introduced to cutting-edge approaches in drug discovery, formulation, development and application, as well as the toxicology of drugs and other xenobiotics.
The program prepares students for careers in the pharmaceutical industry or in the health sciences, whether you are fresh out of your bachelor's degree or already working in the field at the bachelor's level and wish to update your skill set in this rapidly developing field.
Flexibility to Tailor to Your Specialty
While completing a 9-credit hour core curriculum, including Fundamentals in Pharmaceutical Sciences, Applied Statistics, and Ethical Issues, you can tailor your training program with 21 or more credit hours of work in one of five specialty tracks based on your career interests. All specialty tracks are offered in a remote learning or on-campus format:
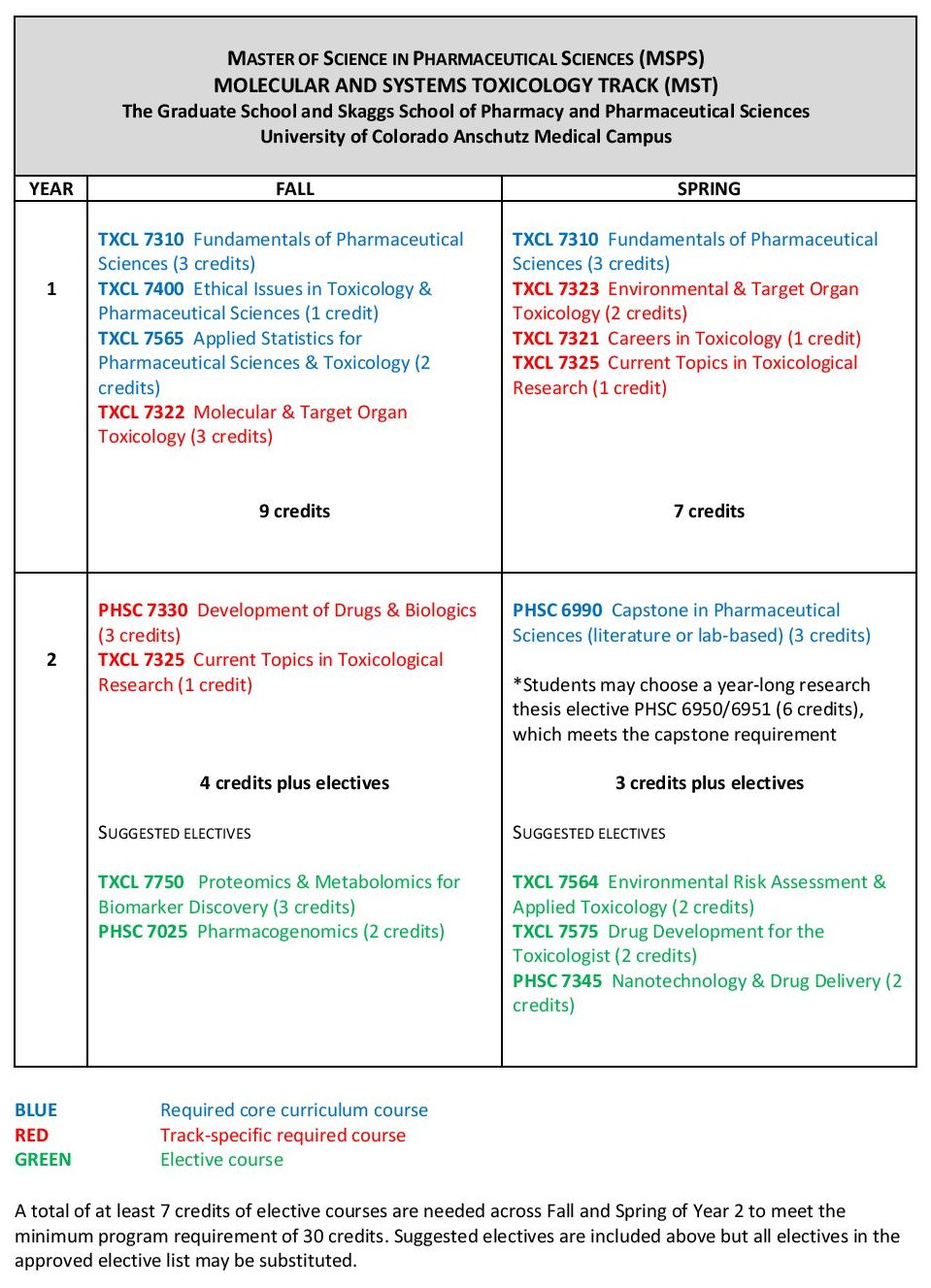
Cannabis Science and Medicine - Advance basic and clinical research on this medicinal plant and its constituents with the goal of improving academic research, industrial practices, and patient outcomes. All cannabis-specific courses are offered online only but students have the option to take other coursework online or on-campus.
Clinical Pharmacokinetics and Pharmacodynamics - Explore factors that influence the concentration of drug at the site(s) of action and examine drug effects and mechanisms of action. This expertise is in high demand in the pharmaceutical industry and clinical research organizations.
Drug Discovery - Learn how the integration of medicinal chemistry with systems approaches, computational and high throughput screening, and bioinformatics has transformed how new drug candidates are identified and advanced. This expertise will position you for jobs in the pharmaceutical industry, academia and government.
Molecular and Systems Toxicology - Learn how the application of systems approaches and the integration of -omics technologies are combined to elucidate the mechanisms underlying the toxic effects of drugs and other xenobiotics. This expertise is sought after by employers in the pharmaceutical and chemical industries, biotechnology and government.
Pharmaceutical Biotechnology and Drug Delivery - Study the development of protein drugs, vaccines and nucleotide-based drugs. This expertise is valuable to the pharmaceutical industry and in start-up biotechnology companies.
The application deadline for Fall 2025 admission has been extended to February 28, 2025.
Applications for all master's and doctoral programs are submitted electronically through the Graduate School of the University of Colorado Denver | Anschutz Medical Campus. After signing up for an account, select 'Master's' under the 'Academic Interests' menu and scroll down to 'Skaggs School of Pharmacy and Pharmaceutical Sciences' and select "MS in Pharmaceutical Sciences."
Application requirements are:
- A completed Graduate School application
- The application fee ($50 domestic, $75 international)
- A bachelor's degree (or equivalent) in biology, biochemistry, chemistry, neuroscience, pharmacy, or a related field from an accredited college or university with a minimum GPA of 3.0
- A written personal statement expressing interest or demonstrated experience, if applicable, in the field of pharmaceutical sciences and the applicant's intended specialty track (i.e., Cannabis Science & Medicine, Clinical Pharmacokinetics and Pharmacodynamics, Drug Discovery, Molecular and Systems Toxicology, or Pharmaceutical Biotechnology and Drug Delivery)
- Three (3) reference letters from persons familiar with the applicant's prior academic performance, potential, character, and suitability for graduate study (using an electronic template generated by the application system)
- Academic transcripts for U.S. applicants: one copy of an official academic transcript must be sent directly by the Registrar of all higher education institutions attended, not just those where degrees were earned. Electronic transcripts are preferred and should be sent to: graduate.school@cuanschutz.edu. If a physical transcript must be sent, request that it be mailed to:
Graduate School
Mail Stop C296
Fitzsimons Building, W5107
13001 E 17th Place
Aurora, CO 80045
- Academic transcripts for international applicants: DO not submit transcripts to the University of Colorado. Instead, applicants with transcripts from institutions outside the United States must order a transcript evaluation from either Educational Credential Evaluators (ECE) or World Education Services (WES). Additional information for international students can be found here at the Office of International Affairs.
Proficiency Exam Requirements:
- Official reports from the TOEFL, IELTS, or DuoLingo English Test are required of applicants whose undergraduate degree was awarded by an institution outside of the United States, United Kingdom, or Canada. For TOEFL, use code 4875. For IELTS or DuoLingo English Test, search "University of Colorado Denver."
- Minimum acceptable English Language Proficiency scores:
- TOEFL - Overall score 90 or higher, with no component below 18
- IELTS - Overall score 7.5 or higher, with no component below 6.5
- DuoLingo English Test - Overall score 120 or higher, with no component below 95
- The GRE (Graduate Record Examination) is not required
Prepare for Tomorrow with One of Five Specialty Tracks in Pharmaceutical Sciences and Toxicology
The multidisciplinary field of pharmaceutical sciences has seen rapid advances that are critical to the discovery and development of drugs for chronic diseases, such as cancer and diabetes, and emerging threats, such as new pathogens and drug resistance. Such conceptual and methodological progress can be difficult to keep up with, even for those already working in the field at the bachelor's level.
To position students for career advancement in this rapidly evolving field, we offer five tracks that help develop leaders in each area. This degree gives students the opportunity to train in the latest advances in pharmaceutical sciences, with approaches and techniques necessary for the development of new drugs, from small molecules and peptides to nucleic acid-based drugs and large, biotechnology-derived molecules.
Why a Master's in Pharmaceutical Sciences?
Adding the MS in Pharmaceutical Sciences to your toolkit will make you more marketable in the field and increase your prospects for employment or promotion. In fact, through 2026, job opportunities in pharmaceutical sciences in the United States and Colorado are expected to increase by 11.4% and 16.5%, respectively – significantly higher than the average for all occupations, according to the U.S. Bureau of Labor Statistics.
Is This Program Right For Me?
Our program is an excellent post-graduate option if you are one of the following:
- Looking to advance your career in the pharmaceutical or biotechnology industry, or academic research, but are time-limited from pursuing a PhD.
- Have a general science background but want to learn how drugs are discovered, developed, and brought to market.
- Interested in establishing a strong foundation for a career in the health professions, including medicine, dentistry, pharmacy or veterinary medicine.
- Interested in exploring a Ph.D. program in pharmaceutical sciences or molecular toxicology.
Why The Skaggs School of Pharmacy and Pharmaceutical Sciences?
Located on the CU Anschutz Medical Campus, our faculty members are nationally and internationally recognized in each of the areas that encompass the field of pharmaceutical sciences. The school is home to the Center for Pharmaceutical Biotechnology, the Center for Translational Pharmacokinetics and Pharmacogenomics, and the Colorado Center for Nanomedicine and Nanosafety. Together, these centers contribute to a wealth of expertise in pharmaceutical sciences.
Graduate Learning Objectives for the MS in Pharmaceutical Sciences
Each graduate degree program (MS, PhD) at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences sets their own Graduate Program Learning Objectives for the students enrolled in each program. These learning objectives are designed by the Graduate Program Committee and Graduate Faculty of the Department of Pharmaceutical Sciences. The MS in Pharmaceutical Sciences program offers a thesis and non-thesis path. While the learning objectives differ between the paths, they are considered equivalent within the same degree.
MS in Pharmaceutical Sciences (Capstone, Non-Thesis Path)
Completion of the curriculum and execution of a final capstone project is the primary path to the MS in Pharmaceutical Sciences at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The MS program learning objectives for the capstone path are:
- Demonstrate knowledge of core concepts in basic and clinical pharmaceutical sciences.
- Critically evaluate the scientific literature.
- Demonstrate proficiency in assimilating and interpreting assigned subject matter.
- Develop basic skills in the responsible conduct of research.
- Formulate hypotheses based on current concepts.
- Design potential research projects.
- Plan data analyses in the context of existing literature.
- Communicate scientific assessments (e.g., literature reviews) effectively through oral presentations and written reports.
- Establish a professional plan for a career in science.
MS in Pharmaceutical Sciences (Thesis Path)
In exceptional cases, a student and faculty member may identify resources for the execution of a research thesis project that meets the capstone requirement. Completion of the curriculum and successful conduct and defense of a master’s thesis is an alternative path to the MS in Pharmaceutical Sciences at the University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences. The MS program learning objectives for the MS thesis path are:
- Demonstrate knowledge of core concepts in basic and clinical pharmaceutical sciences.
- Critically evaluate the scientific literature.
- Demonstrate proficiency in subject matter related to thesis research.
- Demonstrate proficiency in assimilating and interpreting assigned subject matter.
- Develop basic skills in the responsible conduct of research.
- Formulate hypotheses based on current concepts.
- Design and conduct research projects.
- Critically analyze findings in the context of existing literature.
- Communicate research results effectively through oral presentations and written reports and publications.
- Present research results at regional or national meetings, and in peer-reviewed publications or thesis.
- Establish a professional plan for a career in science.
Prerequisites
- B.S. or B.A. in a biological, chemical, or health/medical science,
- be a physician, nurse, pharmacist, physician assistant, or in a public health capacity,
- or
- be a member of other allied health professions (the program director will individually counsel prospective students on any recommended prerequisite coursework)
Course Delivery and Academic Requirements
The online nature of the course and self-directed learning modules will allow flexibility for learners and offer wide geographic engagement.
- Self-directed learning will be complemented by online, synchronous live, case-based discussions and/or activities guided by clinical practice experts, clinical researchers, medicinal plant chemists and pharmacologists, and legal and regulatory leaders.
- 9 credit hours of core coursework in the fundamentals of pharmaceutical sciences, ethics, and biostatistics.
- 15 credit hours of cannabis science & medicine-specific coursework, including endocannabinoid physiology and cannabinoid pharmacology, clinical therapeutics of cannabis and cannabis-derived products, the chemical analysis of cannabis, scientific writing, seminars, and legal and regulatory considerations in cannabis research and therapeutics.
- A minimum of 3 credit hours of elective coursework in drug development, toxicology, pharmacogenomics, or public health topics.
- A final, 3 credit hour capstone project on a cannabis-related project designed by the student and faculty mentor
- The only on-campus course is a 1 credit hour laboratory workshop on cannabis extraction and analytical methods that accompanies the 2 credit hour online course. Students unable to travel to Colorado for the 1 credit hour laboratory component can select an additional elective course
- While the coursework can be completed in as little as four semesters over two academic years, the Graduate School of the CU Anschutz Medical Campus allows up to seven years for completion of a Master of Science degree.
Tuition
- Tuition for this program is $773 per credit hour for Colorado residents and $1,240 per credit hour for out-of-state and international students for a program total of $23,190 (in-state) to $37,080 (out-of-state/international).
- Other fees charged by the Graduate School are updated annually through The Bursar's Office.
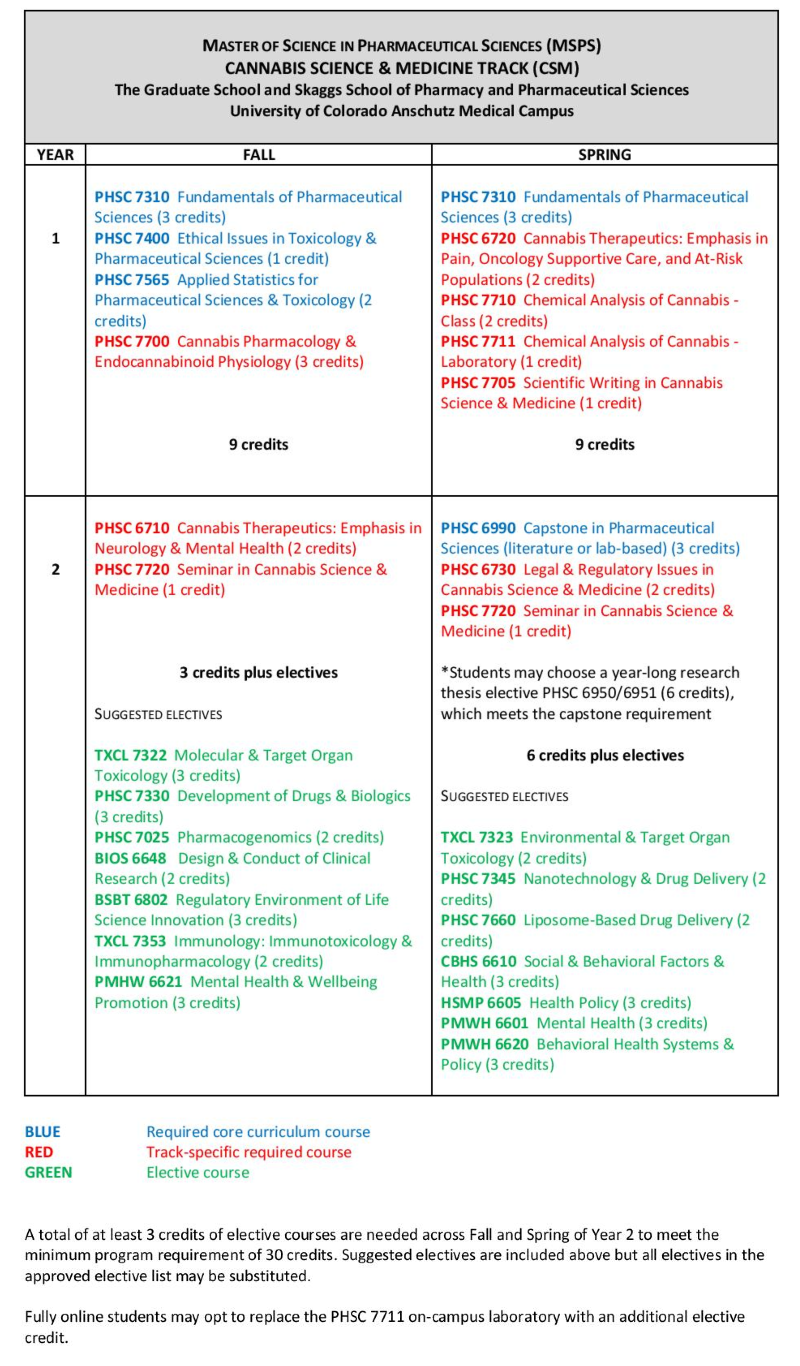
Pharmacokinetics (PK) examines the factors that influence the concentration of a drug at a site of action. PK involves the time-course of the concentration of the drug (and its metabolic products) in the body, including the processes of drug absorption, distribution, metabolism and excretion. Pharmacodynamics (PD) examines drug effects and mechanisms of action.
An understanding of PK and PD is thus critical to every stage of drug development, from pre-clinical research through human clinical trials. Students trained in this track employ equations and models to describe drug concentrations in plasma, blood and other biological samples. The advent of new biotechnology products, combination drug products, drug delivery platforms, and nanotechnology formulations place individuals with PK-PD expertise in high demand for pharmaceutical and medical companies.

Designated as a Center of Excellence for Model-informed Drug Development (MDD)
Our program is designated as a Center of Excellence by Certara, a global leader in biosimulation. We are one of only five pharmacy schools in the nation to receive this designation which allows our students to use Certara’s Phoenix software suite as part of their analyses and method development.
Sample curriculum for Clinical Pharmacokinetics and Pharmacodynamics track
Courses listed in blue are core classes required for all tracks; courses in red are required for the specific track; courses in green are representative electives (specific selections will be determined by the advisor and student based on career goals).
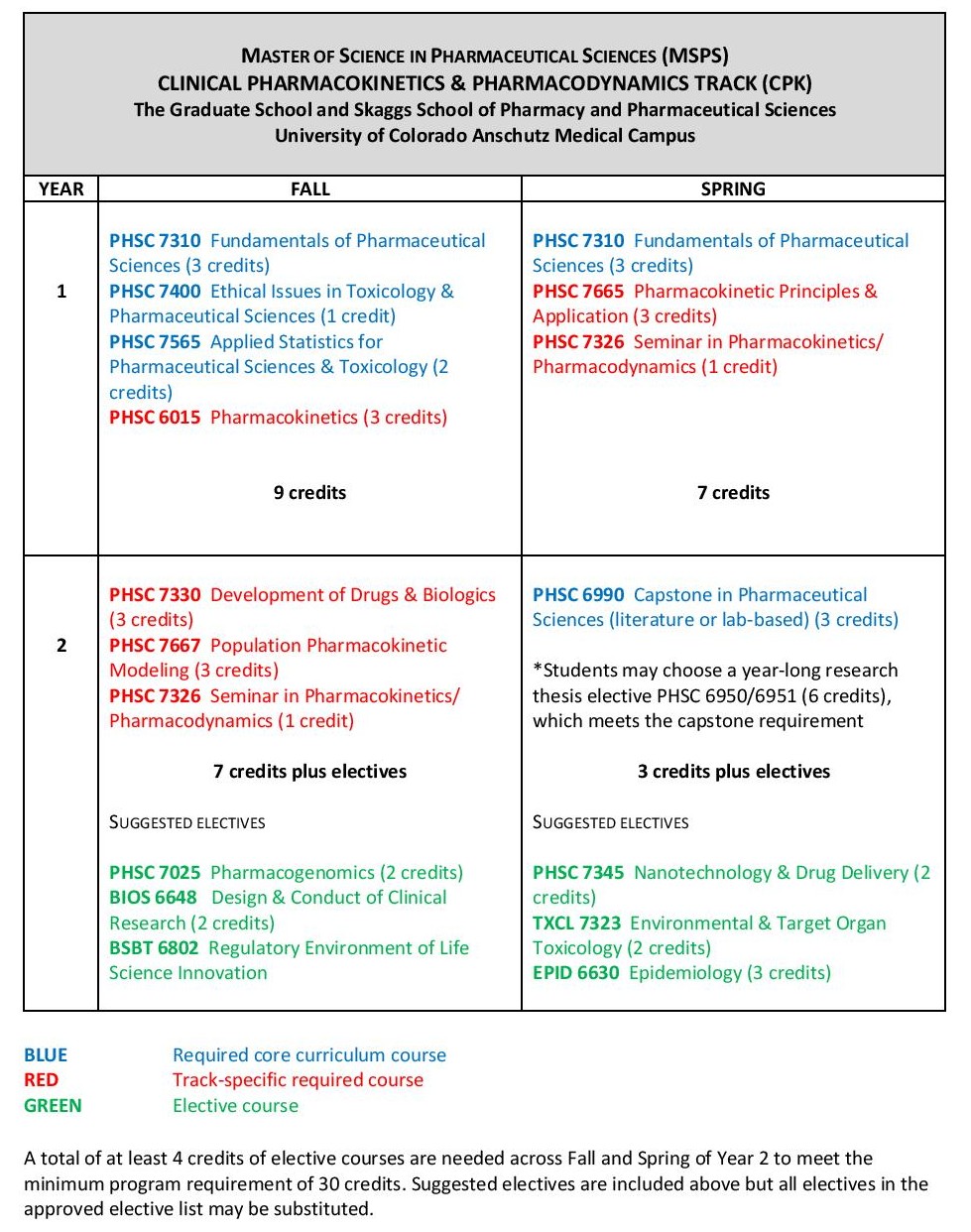
Modern drug discovery has been transformed by the integration of medicinal chemistry with systems approaches, computational and high throughput screening, and bioinformatics. New therapeutics are designed to be highly selective and are targeted to individuals or groups of patients. Transforming drug targets into therapeutics is fast becoming the new standard in discovery efforts.
This track offers you an opportunity to gain insight and experience in the drug discovery process. This includes computational design of molecules, high throughput/high content screening, structure-activity relationships, the selection of appropriate biomarkers for drug action, targeting drugs for personalized therapies, and the application of bioinformatics in the overall drug discovery process. Students trained in these approaches are well-positioned for jobs in the pharmaceutical industry, academia, and governmental regulatory bodies.
Sample curriculum for the Drug Discovery track
Courses listed in blue are core classes required for all tracks; courses in red are required for the specific track; courses in green are representative electives (specific selections will be determined by the advisor and student based on career goals).
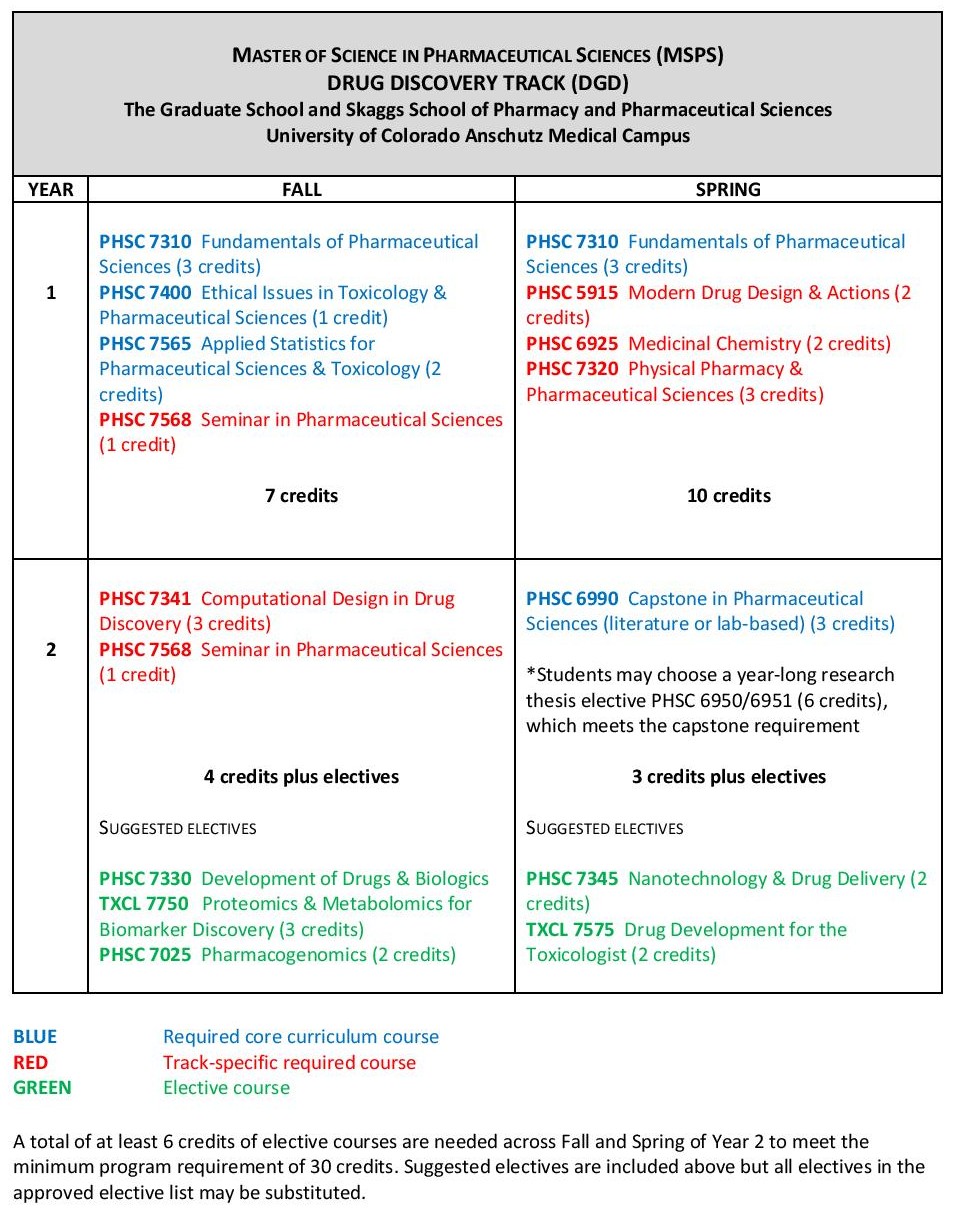
Like many other disciplines, mechanistic toxicology has been transformed by the application of systems approaches and the integration of genomic, transcriptomic, proteomic and metabolomic techniques to elucidate molecular mechanisms of toxicity. Future toxicologists must be able to harness and exploit the power of molecular and systems approaches to reveal the mechanisms underlying the toxic effects of drugs and other xenobiotics.
This track affords you the opportunity to learn about systems toxicology and receive the training necessary to succeed in a changing research environment that is rapidly becoming focused on big data. Students graduating from this track will be sought after by employers in industry, biotechnology and government.
Sample curriculum for the Molecular and Systems Toxicology track
Courses listed in blue are core classes required for all tracks; courses in red are required for the specific track; courses in green are representative electives (specific selections will be determined by the advisor and student based on career goals).
Biologic-based drugs have revolutionized the treatment of cancer and other immune- and inflammatory-based diseases, such as asthma and rheumatoid arthritis, and thus represent an increasing proportion of the therapeutic tools used for those conditions. Pharmaceutical biotechnology and drug delivery are concerned with the development of protein drugs, vaccines and nucleic acid-based drugs.
This track will provide you with the fundamental knowledge required for the synthesis, characterization, formulation, stabilization and delivery of these drugs. By possessing a sound understanding of how to successfully develop and deliver a biotechnology drug, students graduating from this track will be recruited by the pharmaceutical industry or new start-up biotechnology companies.
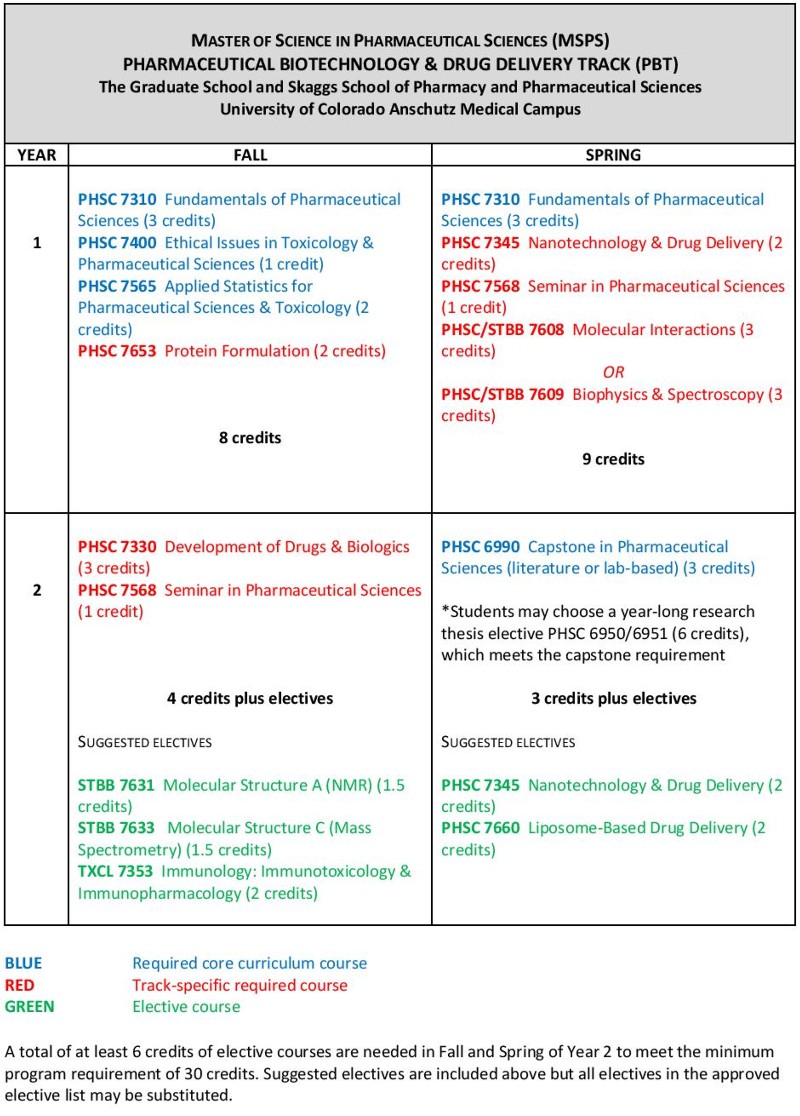
Sample curriculum for the Pharmaceutical Biotechnology and Drug Delivery track
Courses listed in blue are core classes required for all tracks; courses in red are required for the specific track; courses in green are representative electives (specific selections will be determined by the advisor and student based on career goals).

David Kroll PhD
Professor
Master’s Degree and Certificate Programs in Pharmacy & Pharmaceutical Sciences Director
- Department of Pharmaceutical Sciences
Email Address:david.kroll@cuanschutz.edu
Primary Phone:303-724-4626
Mailing Address:
- CU Anschutz
Pharmacy and Pharmaceutical Sciences Building
12850 East Montview Boulevard
Office: V20-2119
Aurora, CO 80045
Dr. David Kroll returned to the CU Skaggs School of Pharmacy and Pharmaceutical Sciences in January, 2019, as Professor of Natural Products Pharmacology & Toxicology and Director of Master’s Degree and Certificate Programs in Pharmacy and Pharmaceutical Sciences.
Dr. Kroll began his independent academic career here in the Department of Pharmaceutical Sciences in 1992 as Assistant Professor of Pharmacology & Toxicology, and was promoted to Associate Professor with tenure in 1999. He then conducted sabbatical research during the 2000 calendar year in the Department of Microbiology and Immunology at Duke University in Durham, NC. That year, Dr. Kroll also became the first School of Pharmacy faculty member to be elected to the system-wide CU President’s Teaching Scholar Program.
After returning to CU in 2001, family medical issues led him to relocate to North Carolina where he was Senior Research Pharmacologist in the Natural Products Laboratory at Research Triangle Institute (RTI International) until 2008. Dr. Kroll then became Professor and Chair of the Department of Pharmaceutical Sciences at North Carolina Central University in Durham, an historically Black college/university (HBCU) in the University of North Carolina system, until the end of 2011. Dr. Kroll’s laboratory was continuously funded by research grants from the National Institutes of Health or the American Cancer Society during this time, where his team investigated plants, bacteria and fungi for novel anticancer drugs and dietary supplements that might interact with those drugs.
Dr. Kroll’s interest in public science communication and service to the MS in Medical and Science Journalism program at the University of North Carolina at Chapel Hill from 2004 to 2012 led him to transition into freelance and institutional science writing and public science engagement as Director of Science Communication at the then-new Nature Research Center of the North Carolina Museum of Natural Sciences in Raleigh. His position there from 2012 through 2014 was jointly supported by the Department of English at North Carolina State University, where he taught graduate courses in science and environmental writing and an undergraduate course in the basic principles of news reporting.
Outside of academia, Dr. Kroll was also an early science blogger, with his Terra Sigillata natural products pharmacology blog joining the ScienceBlogs network in 2006, named that year by Nature as one of the top 50 blogs worldwide written by practicing scientists. His blogs have also been hosted by the American Chemical Society and PLOS.
From 2014 to 2018, Dr. Kroll devoted himself exclusively to full-time freelance medical journalism with clients that included Reuters, the American Chemical Society’s Chemical & Engineering News (C&EN), and the Research Triangle’s alt-weekly, INDY Week. Dr. Kroll was also a regular contributor from 2011 to 2018 to the Pharma, Healthcare & Innovation section of Forbes.com. Throughout his 30-plus-year career, Dr. Kroll has connected with general audiences through multiple appearances on the NPR radio show, The People’s Pharmacy with Joe & Terry Graedon, and in his service as an expert source to ABC World News Tonight, NBC News, CNN’s Don Lemon Tonight, The Denver Post, The (Raleigh, NC) News & Observer, The Colorado Sun, and television stations across the US, Canada, and China.
The opportunity to return to the CU Skaggs School of Pharmacy and Pharmaceutical Sciences in 2019 now allows Dr. Kroll to meld his passion for graduate and professional education with the skills he gained in communicating complex drug and medical topics to general audiences. Dr. Kroll’s background in natural products pharmacology contributed to the school’s development of several educational programs for scientists and health care practitioners on medical cannabis and the risks and potential benefits of products made from the medicinal plant.
Throughout his career, Dr. Kroll’s philosophy has been that all scientists and health care professionals have responsibilities not only to their professional communities, but to society at-large, engaging with citizens and other diverse stakeholders to navigate through the complex social and economic issues of health and wellness in global society.
Education, Licensure & Certifications
- BS, Toxicology (1985), Philadelphia College of Pharmacy & Science
- PhD, Pharmacology & Therapeutics (1989), University of Florida College of Medicine
- Postdoctoral Fellowship (1990-1992), Endocrinology & Medical Oncology, University of Colorado School of Medicine
Affiliations
- Master of Science in Pharmaceutical Sciences Program
- PhD in Pharmaceutical Sciences Program
- Cannabis Science & Medicine Graduate Certificate
- PhD in Toxicology Program