Immunotoxicology Laboratory - Dr. Jared Brown
Research in the Brown lab is focused on immune responses to particulates including nanoparticles, air pollution, silica and coal dust. In particular, our research group investigates the role of innate immune cells, including mast cells and macrophages, in response to particulates and their role in disease. In addition, we are interested in understanding the toxicity of engineered nanomaterials as well as their use in drug delivery and treatment of cancer. Lastly, we are interested in understanding the contribution of environmental contaminants in the development of chronic kidney disease of unknown origin (CKDu).
Jared Brown PhD
Professor
Toxicology Graduate Program Director, T32 Training Program in Molecular and Systems Toxicology Director, Colorado Center for Nanomedicine and Nanosafety Co-Director
Phone:303-724-8213
Research in the Brown lab broadly focuses on immune responses to environmental and occupational exposures. Our research ranges from understanding immune responses to nanoparticles and air pollution to chemical warfare agents and occupational exposures. In addition, we are interested in developing novel nanotherapeutics for treatment of cancer and allergic disease. Examples of current projects in the Brown lab are described below:
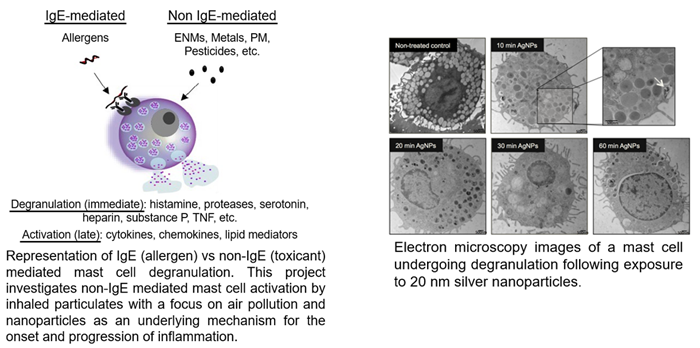
1. Understanding mechanisms of non-IgE mast cell activation by environmental particulates.
This work, funded by NIEHS R01 ES019311, is examining novel mechanisms by which nanoparticles and airborne particulate matter trigger non-IgE mast cell activation contributing to adverse pulmonary and cardiovascular outcomes. Specifically, we are investigating redox and non-redox mechanisms linked with thioredoxin interacting protein and its potential regulatory role in mast cell degranulation. In addition, we are interested in cellular metabolism changes which occur between IgE and non-IgE mast cell degranulation that may provide insight into disease mechanisms in mast cell activation disorders. Lastly, we are working with Drs. Stephen Dreskin and Jenny Stitt from the Anschutz School of Medicine to examine these mechanisms of non-IgE mast cell degranulation in patients with chronic idiopathic urticaria.
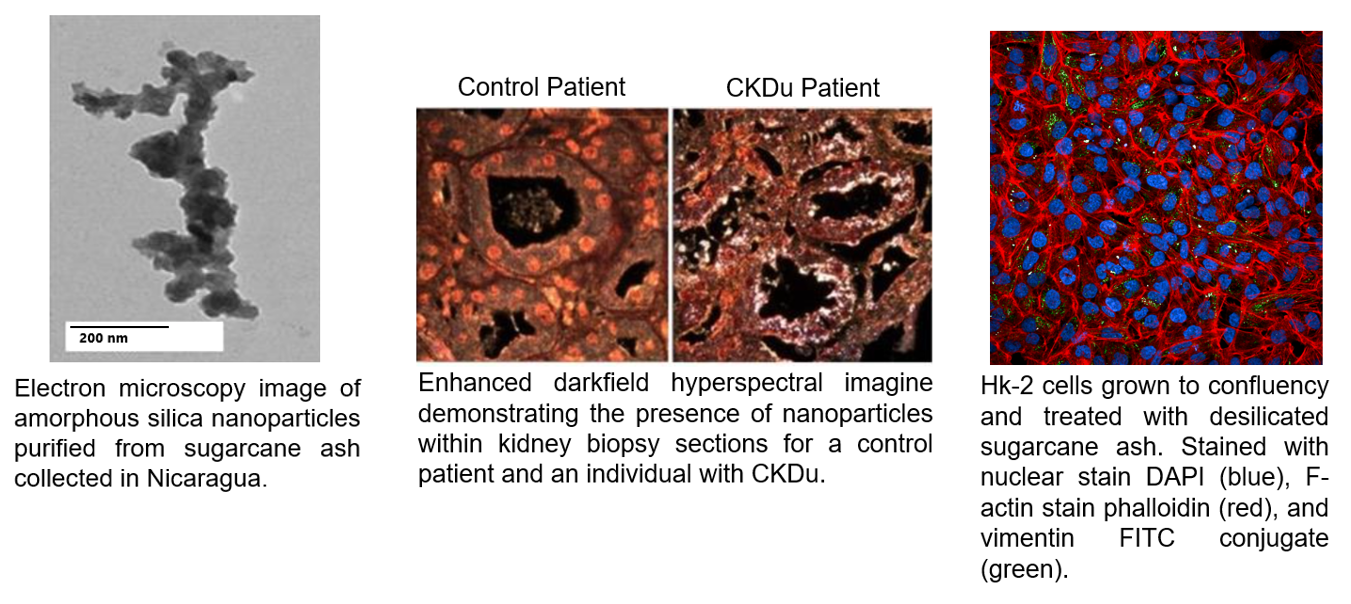
2. Silica Nephropathy and Chronic Kidney Disease of Unknown Etiology.
This work, funded by NIDDK R01 DK12351 and in collaboration with Drs. Richard Johnson and Carlos Roncal within the School of Medicine, is examining the contribution of inhaled silica nanoparticles produced during burning of sugarcane fields in the development of chronic kidney disease of unknown etiology (CKDu). CKDu is worldwide epidemic largely associated with coastal agricultural workers leading to chronic kidney disease in young individuals and often resulting in kidney transplant or death. There are many potential contributing factors including heat stress, dehydration and environmental exposures. We are currently examining kidney biopsy sections from CKDu patients for the presence of silica nanoparticles that are found in high abundance in the ash from sugarcane burning. We are using two novel techniques to identify silica nanoparticles in biopsy sections: 1) single particle inductively coupled plasma mass spectrometry to provide size and chemical information on the silica nanoparticles and 2) enhanced darkfield hyperspectral imaging to image nanoparticles in tissue. In addition, we are using a human proximal convoluted tubule cells and animal models to investigate potential mechanisms of toxicity by sugarcane ash and purified amorphous silica nanoparticles.
3. Contribution of mast cells to nitrogen mustard pulmonary and central nervous system toxicity.
This work, funded by the NIEHS and the Department of Defense and working with Dr. Neera Tewari-Singh (Michigan State University), is investigating the role of mast cells in mediating inflammation in response to exposure to sulphur mustard and phosgene oxime, both of which are chemical warfare agents. We have utilized a mouse model of mast cell deficiency to demonstrate that in the absence of mast cells that inflammation resulting from sulphur mustard exposure is largely diminished. In addition, we are examining the effects of mast cell activation in the brain following sulphur mustard exposure. Lastly, we developing a nanoparticle based therapeutic to prevent mast cell activation as a prophylactic treatment for military personnel and civilians to prevent the effects of chemical warfare agents.
.tmb-image350.png?Culture=en&sfvrsn=55c507bb_1)
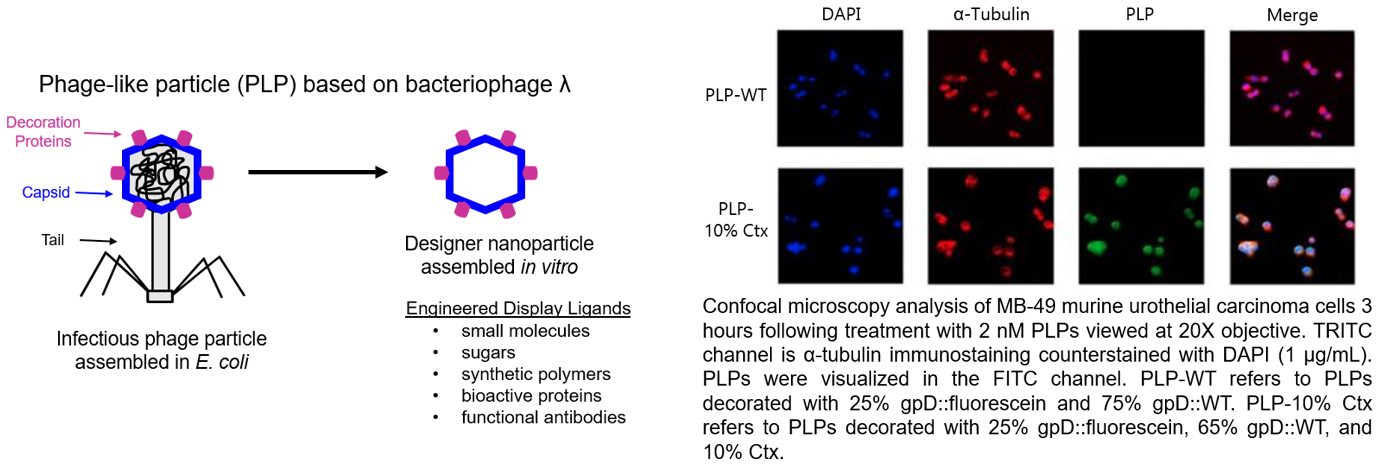
4. Development of designer nanoparticle for treatment of bladder cancer.
This project is funded by the Department of Defense and is in collaboration with Drs. Carlos Catalano and Tom Flaig. Our goal is to develop a novel bacteriophage like nanoparticle (Phage like particle, PLP) for the treatment of bladder cancer. The PLP is targeted to bladder cancer cells and designed to deliver an agonist of the STING pathway to activate an anti-tumor immune response through production of type 1 interferons.
5. The effect of coal and mine respirable dust on lung cells and exposure assessment.
This project, funded by the Alpha Foundation, and in collaboration with Drs. Candace Tsai (UCLA) and Jurgen Brune (Colorado School of Mines) is investigating the contribution of nano-sized fraction of coal dust to lung disease. There has been an increase in lung disease in coal miners in recent years due to changes in mining practices. We have hypothesized that there is an increase in generation of nano-sized coal dust that contributes to the increase in lung disease. We are using an air-liquid interface of human lung epithelial cells to investigate various nano-sized fractions of coal dust toxicity.
6. Inductively coupled plasma mass spectrometry studies:
We have a number of funded collaborations with various investigators including Drs. Anne Starling (CSPH), Kathy James (CSPH), James Roede to measure metal levels in various human, animal and cell samples. We have also developed a single particle ICP-MS method to measure nanoparticles in tissue and cells and have the capability to speciate metals such as arsenic.
Arthur Stem
Graduate Student
Arthur.Stem@CUAnschutz.edu
Arthur is investigating the mechanisms by which amorphous silica nanoparticles from sugarcane ash contribute to chronic kidney disease of an unknown etiology. Specifically Arthur is determining the role of altered cellular metabolism caused by exposure to silica nanoparticles or sugarcane ash along with understanding the role of reactive oxygen species in cellular toxicity.

Angela Reinert
Graduate Student
Angela.Reinert@CUAnschutz.edu
Angela is investigating the pathways involved with IgE-independent mast cell activation. Specifically, she is interested in the orphan receptor, Mas-Related G-Protein Coupled Receptor X2 (MRGPRX2), and its role in activation of mast cells by environmental toxicants.

Emily Ruggiano, MS
Graduate Student
Emily.Ruggiano@cuanschutz.edu
Emily is exploring the role of thioredoxin interacting protein (TXNIP) in regulating redox and non-redox mechanisms of mast cell activation upon exposure to environmental pollutants.

Matthew Gibbs, MS, PhD
Post-Doctoral Fellow
Matthew.Gibb@cuanschutz.edu
Matthew has previously worked in pulmonary immune responses to infectious diseases, and recently finished his PhD in pulmonary immunotoxicology where he worked on using in vitro models to identify and differentiate respirable sensitizers from non-sensitizers. Currently, he is investigating pathways and mechanisms involved in CKDu from environmental exposures as well as looking into the role mast cells play in response to inhaled chemical warfare agents.

Stephen Brindley, MS
Professional Research Assistant III
Stephen.Brindley@cuanschutz.edu
Stephen runs the ICP-MS instrument where he quantifies metals in prenatal vitamins and analyzes levels of various nanoparticles in different biological matrices. Stephen also works on a coal related project evaluating if particle size plays a role in coal toxicity.

Robert Canfield
Graduate Student
Robert.Canfield@CUAnschutz.edu
Bob is helping develop a phage like nanoparticle for treatment of bladder cancer. Specifically, he is investigating activation of the STING pathway to induce an anti-tumor immune response by the phage like nanoparticles.

| Lab Alumni | Current Position |
| Diksha Diundi, MS | Regulatory Fellow at Synterex |
| Yanhao Jiang, MS | Graduate Student in Pharmaceutical Sciences at University of Arizona |
| Keegan Rogers, Ph.D. | Health Scientist at Stantec Chemrisk |
| Angela Cruz-Hernandez, Ph.D. | Senior Scientist in Product Safety at L'Oreal |
Ryan Mendoza, Ph.D. | Senior Scientist, IgM Biosciences |
| Dylan Fudge, Ph.D. | Molecular Toxicologist, U.S. Army DEVCOM Chemical Biological Center |
| Nasser Alsaleh, Ph.D. | Associate Professor, King Saud University |
| Indushekhar Persaud, Ph.D. | Principal Investigator, Incyte Pharmaceuticals |
| Valerie Minarchick, Ph.D. | Research Associate, University of Colorado Anschutz Medical Campus |
| Jonathan Shannahan, Ph.D. | Associate Professor, Purdue University |
| Monica Johnson, Ph.D. | Co-Founder, STEM Boomerang, New Mexico |
| Wei Bai, Ph.D. | Owner, SilkRdConnect, Scientific Communications |
| Ramakrishna Podila, Ph.D. | Associate Professor, Clemson University |
| Sky (Xiaojia) Wang, Ph.D. | Professor, Beaufort Community College |
| Abdullah Aldossari, Ph.D. | Assistant Professor, King Saud University |
| Pranita Kabadi, Ph.D. | Associate Director, Patient Safety, AstraZeneca |
